2022-2023 Research Updates
Research to improve the quality of life of our community remains a commitment for us as an organization. We do this work through The Pauline H. Siegel Eclipse Fund for Research and the James T. Lubin Clinician-Scientist Fellowship Program.
We are excited to share some of the research advances with you.
Understanding Causation
Genetics and Biomarkers
In 2022, Dr. Monique Anderson was awarded a research grant to continue research with Dr. Michael Levy focused on genetics and diagnosis of transverse myelitis. In his previous work, Dr. Levy discovered a mutation (or change) in the VPS37A gene in eight patients with transverse myelitis. VPS37A makes a protein that is important for recycling and removing proteins from cells. Some of these proteins are placed in the space outside of cells into sacs, some of which are called exosomes. Dr. Levy and Dr. Anderson aren’t yet sure how changes in this process are involved in transverse myelitis. Changes in this gene may cause proteins to be taken outside of the cell when they aren’t supposed to be. The immune system then might react to this disruption in a way that causes inflammation of the spinal cord. Interestingly, this mutation only seems to increase someone’s risk of monophasic transverse myelitis, meaning these patients only have one attack.
Dr. Anderson’s study is investigating if new markers within exosomes are found in the blood of patients with transverse myelitis. These markers could potentially be used in the diagnosis of transverse myelitis. The researchers also hope to better understand the signaling pathways between cells that trigger an immune response against the spinal cord. The study includes both patients with idiopathic transverse myelitis (meaning no cause has been found yet) and patients with multiple sclerosis (MS), myelin oligodendrocyte glycoprotein antibody disease (MOGAD), and neuromyelitis optica spectrum disorder (NMOSD). Dr. Anderson hopes to determine if there are any shared mechanisms between these diseases and if there are similar proteins within exosomes that the immune system responds to.
In an “Ask the Expert” podcast episode with SRNA, Dr. Anderson explains that, “if there are similar mutations occurring in the different patient populations, that might give us a clue of something that’s…occurring in these similar diseases. So, it gives us an idea of, one, are there additional genetic tests that we should be doing for patients? And two, is this a potential therapeutic target for these patients?” Dr. Anderson also highlights that the signaling pathway being studied is relevant to other neurologic diseases and that she hopes the study will guide future transverse myelitis therapies. In her words, “…that same … pathway has been implicated in several other neurologic disorders, especially in the realm of repair and recovery. So, it’s unclear if this is also going to be the case with transverse myelitis, but this is something that we’re hoping to answer as well.”
In 2023, Drs. Anderson and Michael Levy received an NIH R01 award of $1.25 million to dig deeper into this genetic mutation and potentially identify new targets for therapy. Better understanding how diseases such as transverse myelitis happen will improve our ability to diagnose, treat, and perhaps prevent them.
*Excerpted from a blog written by Hannah Kelly, a volunteer for the Siegel Rare Neuroimmune Association.
Causes of Myelopathy
In 2022, former SRNA James T. Lubin Fellow Dr. Olwen Murphy published an article in the Journal of Neurological Sciences, “Identification of specific causes of myelopathy in a large cohort of patients initially diagnosed with transverse myelitis” with her mentor Dr. Carlos Pardo. This research identifies the etiologic diagnosis in individuals presenting with myelopathy to the Johns Hopkins Myelitis and Myelopathy Center between 2006 and 2021 for evaluation of “transverse myelitis.” The final diagnosis was determined after a comprehensive evaluation of each patient and was reviewed and validated. Of 1193 included patients, 772 (65%) were determined to have an inflammatory myelopathy, and 421 (35%) were determined to have a non-inflammatory myelopathy. Multiple sclerosis/clinically isolated syndrome (n = 221, 29%) and idiopathic myelitis (n = 149, 19%) were the most frequent inflammatory diagnoses. Spinal cord infarction (n = 197, 47%) and structural causes of myelopathy (n = 108, 26%) were the most frequent non-inflammatory diagnoses. Compared to individuals with inflammatory myelopathies, those with non-inflammatory myelopathies were more likely to be older, male and experience chronic symptom evolution. Hyperacute onset (less than six hours from their symptom onset to their worst neurological symptoms) was most frequent in those with spinal cord infarction (74%). In contrast, chronic symptom evolution was most frequent in individuals with structural causes of myelopathy (81%), arteriovenous fistula or arteriovenous malformation (81%), myelopathy associated with rheumatologic disorders (71%), and sarcoidosis-associated myelopathy (61%). They found that most of those initially diagnosed with “transverse myelitis” were eventually found to have a more specific inflammatory or even non-inflammatory cause. This could potentially lead to inappropriate treatment.
Improving Diagnosis
Assessing Condition-Specific Knowledge in Patients with Rare Neuroimmune Disorders
In 2022, SRNA awarded an Eclipse Fund Research Grant to former SRNA James T. Lubin Fellow Dr. Kyle Blackburn, Assistant Professor in the Department of Neurology at UT Southwestern Medical Center for “Assessing Condition-Specific Knowledge in Patients with Rare Neuroimmune Disorders (RNDs).” This study involves two phases. In the first phase, a measure of condition-specific knowledge of RND (with questions on RNDs broadly as well as a subset of questions for each disorder) was developed by a multidisciplinary clinicians and team members of the Siegel Rare Neuroimmune Association (SRNA). This measure contains questions to assess participant knowledge of the diagnosis of RND symptoms and prognosis. The measure was field tested in a small sample of patients at UT Southwestern Medical Center. Based upon feedback, the instrument was revised.
During an upcoming second phase, the new instrument will be assessed in patients with RND (estimated n=100). The primary endpoint of the study is to determine the condition-specific knowledge in those with RND. The data collected in this study will be utilized to identify knowledge gaps in this population, which will inform future educational efforts.
Common Variable Immunodeficiency and Sjogren’s Syndrome
Former James T Lubin Fellow and assistant professor of neurology at the University of Utah, Dr. Jonathan Galli, published two articles in neurology journals in 2023 with his mentor Dr. Stacey Clardy. You can find “Neurologic Manifestations of Common Variable Immunodeficiency: Impact on Quality of Life” in Neurology: Neuroimmunology and Neuroinflammation, and “Neurologic involvement in seronegative primary Sjögren’s syndrome with positive minor salivary gland biopsy: a single-center experience” in Frontiers in Neurology.”
NMOSD Clinical Record Review
In 2023, SRNA’s Director of Research and Programs, GG deFiebre, was a co-author on two papers published in the Journal Neurology and Therapy.
The first paper, “Understanding Treatment Decisions in Neuromyelitis Optica Spectrum Disorder: A Global Clinical Record Review with Patient Interviews,” sought insights into neuromyelitis optica spectrum disorder (NMOSD) treatment practices worldwide. Neurologists from the USA, Germany, Italy, Brazil, South Korea, and China completed an online survey and submitted clinical records for aquaporin-4 (AQP4) immunoglobulin G (IgG)-seropositive adults with NMOSD, which included patient demographics, diagnosis, maintenance treatment history, relapse occurrence, and severity. Patients receiving NMOSD maintenance therapy were interviewed about their diagnosis, treatment, perceptions about relapse severity or disease stability, and treatment switches. A total of 389 neurologists submitted clinical records for 1185 patients with AQP4-IgG-seropositive NMOSD and 33 patients with NMOSD were interviewed. Approximately 25% (228/910) of patients from the clinical record review (CRR) were initially misdiagnosed and 24% (8/33) of patients interviewed reported misdiagnosis. Misdiagnosis was associated with treatment delay and more relapses compared with those receiving the correct initial diagnosis (mean 3.3 vs 2.8). Maintenance therapy was not initiated within 2 months for 47% (221/472) of patients from the CRR and 24% (8/33) of interviewed patients. Oral corticosteroids/immunosuppressive therapies were typically the first maintenance treatment initiated, except for the USA, where monoclonal antibodies were equally likely to be prescribed. Relapse severity influenced the decision to initiate/change therapy and use monoclonal antibodies. Of the interviewed patients, 76% (25/33) did not recall having a choice of treatment and many did not know the rationale for treatment choice.
The second paper, “Characterization of Disease Severity and Stability in NMOSD: A Global Clinical Record Review with Patient Interviews,” sought insights into the classification of and factors associated with relapse severity and disease stability in NMOSD clinical practice worldwide. The same data as the prior study showed that there was no clear consensus on how relapse severity was defined in clinical practice, with geographical variations in relapse classification. Neurologists tended to rely on clinical assessments when determining severity, viewing each relapse in isolation. In contrast, patients had a more subjective view based on the changes in their daily lives and comparisons with prior relapses. Similarly, there was a disconnect in the definition of disease stability in that the complete absence of relapses was more important for patients than for neurologists.
These studies were sponsored by F. Hoffmann-La Roche.
Restoring Function
The Study to Investigate the Safety of the Transplantation of Human Glial Restricted Progenitor Cells Into Subjects With Transverse Myelitis at UTSW Medical Center in Dallas is the first to explore the use of stem cells for repairing spinal cord damage in transverse myelitis. These stem cells are capable of developing into cells that produce myelin when placed into human tissue. In research studies, it has been shown that after being transplanted into the spinal cords of individuals with transverse myelitis, these cells will produce myelin. Preclinical studies in animals have demonstrated safety and efficacy of the stem cells. The FDA has approved the surgical device used to implant these stem cells into the spinal cord. Following a delay caused by the COVID-19 pandemic, the first individual was enrolled in December of 2022 and the second in September 2023.
“Where we are today has literally been 20 years in the making. And so, the steps to get to a clinical trial can be complicated, and for cell-based therapies, they’re particularly complicated.” – Dr. Benjamin Greenberg, Vice Chair for Clinical and Translational Research, Department of Neurology, University of Texas Southwestern.
If the trial demonstrates that the procedure is safe and effective, the investigators will seek approval to enroll more people with TM. The plan is to enroll up to nine patients in this study. Candidates for the trial undergo a thorough review of their medical records and imaging before visiting UT Southwestern for screening appointments, ensuring they meet the inclusion criteria.
The surgery involves implanting stem cells into the spinal cord, followed by a short hospital stay. Afterwards, patients are monitored in outpatient clinics over a period of several months to ensure no toxicity occurs from the stem cells or from the immunosuppressive therapies required to prevent an adverse immune reaction to the stem cells.
Currently, the primary goal of this Phase 1/2a study is to assess the procedure’s safety and to establish the appropriate dosage. The next phase, Phase 2b, will involve a larger number of individuals to further evaluate the procedure’s safety and efficacy. Since transverse myelitis is a rare and serious condition, it is hoped that the FDA will grant approval for the therapy before conducting later-stage trials. For additional insights, explore this 2023 RNDS video detailing the study.
*Excerpted from a blog written by Hannah Kelly, a volunteer for the Siegel Rare Neuroimmune Association.
Progress Grant for NMOSD
Dr. Sammita Satyanarayan continued work on the research funding she received as part of the Progress Grant for NMOSD. The Progress Grant funds research aimed to improve the understanding of neuromyelitis optica spectrum disorder (NMOSD), specifically focused on Asian and African American populations.
Dr. Sammita Satyanarayan is conducting research on “Assessing the impact of social disparities of health on disability and access to care in NMOSD patients.” She is a neuroimmunology fellow at the Mt. Sinai Hospital in New York City, NY. Her study is focused on evaluating how the factors of people’s lives, called social determinants of health, impact their access to care, disability, and disease process in people with NMOSD.
Some of the social determinants of health Dr. Satyanarayan is studying include education access and quality, health care access and quality, neighborhood and built environment, social and community context, and economic stability. Differences in these factors can lead to differences in the disease process, which are called disparities. A better understanding of these factors can help medical professionals address disparities while providing care to individuals diagnosed with NMOSD.
Access to care can be measured by the time between symptom onset to diagnosis, the time it takes to receive the first disease-modifying treatment, and the type of disease-modifying therapy received. Disability, on the other hand, is measured by patient-reported outcomes, such as ambulation status and activities of daily living; physician-reported status, such as ambulation status; standardized and validated disability scores, such as the Expanded Disability Status Scale (EDSS) and the Timed 25-Foot Walk; and examination of vision and visual acuity. Dr. Satyanarayan’s study will include both prospective data (data focused on the present and future) and retrospective data (data focused on the past) and will include data from three academic medical centers – Mt. Sinai Hospital, University of Southern California, and Massachusetts General Hospital. You can learn more about the research study by viewing Dr. Satyanarayan’s presentation at the 2021 Rare Neuroimmune Disorders Symposium (RNDS).
We look forward to learning more about the impact of health disparities in people with NMOSD from Dr. Satyanarayan’s research, and we are hopeful that it will lead to better care for those in the NMOSD community.
SRNA James T. Lubin Fellowship
In addition to some of the research described above, we remain committed to funding Fellowships to train clinician-scientists in rare neuroimmune disorders.
In 2023, Dr. Haiwen Chen started her fellowship at Johns Hopkins University under the mentorship of Dr. Carlos Pardo. Dr. Chen is a pediatric neurologist and neuroscientist who received her medical degree from the University of Maryland. She then completed a Pediatrics Residency at The Johns Hopkins Hospital in Baltimore, Maryland. She is interested in understanding how MOGAD affects neurons and oligodendrocytes to cause neurological dysfunction. To do so, she is developing an in vitro model system of neurons and glia to assay how they are disrupted by MOGAD autoantibodies and the associated inflammatory response, where macrophages, T-cells, B-cells, complement, and microglial activation through cytokine release have all been implicated.
Understanding this process may help us comprehend how MOGAD causes brain and spine dysfunction. We can then intelligently design better strategies to treat the disease. For example, understanding what part of the immune system may be overactive in MOGAD may allow us to selectively target and suppress that particular component of the immune system to mitigate disease. Furthermore, the team will use immunologic and metagenomic technologies to look for concurrent infections in patients with MOGAD to investigate whether infections may drive immune reactions initially intended to fight off infection that then also attacks the body’s own cells, causing autoimmune disease i.e. MOGAD. They postulate that the type of trigger for disease along with the characteristics of the autoantibody in individual patients, such as where it binds and how strongly it binds, may provide insight into the clinical course of disease. Finally, the team will investigate why patients develop different severity of disease, different rates of relapse, and different responsiveness to treatments. They predict that these clinical features may be related to how much brain cell dysfunction is caused by the disease as well as how the disease is triggered. Better understanding of how these clinical differences arise will allow us to be better able to counsel patients on prognosis and course of illness. Moreover, predictors for severity of clinical course would likely affect management choices in terms of how aggressively to treat disease to minimize neurological disability while balancing possible risks of different treatment courses.
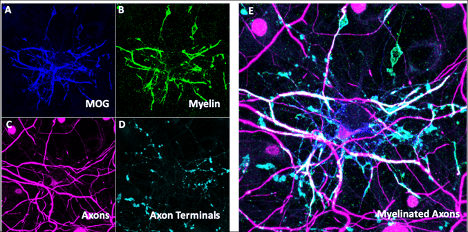
Figure Legend: In vitro model of myelinated neurons. Immunohistochemistry showing presence of oligodendrocytes labeled by MOG (A) and MBP (B) and neurons labeled with NF-H (C) and vGlut1 (D) with composite image (E) showing their overlap which represents myelinated segments of the axons of neurons.
SRNA-Led Research
SRNA Registry
The goal of the SRNA Registry that was started in 2016 is to help advance research about rare neuroimmune disorders, collaborate with researchers from around the world, and identify participants for clinical trials.
As of December 2023, 711 people have participated in the registry. We also recently added a longitudinal component to the registry which participants will be asked to fill out on a yearly basis. This component is important because people’s diagnosis sometimes changes, and we want to measure if there any changes in someone’s medications and their quality of life.
As of December 2023, 68% of respondents were diagnosed with transverse myelitis, 10% with neuromyelitis optica spectrum disorder, 4% with acute disseminated encephalomyelitis, 4% acute flaccid myelitis, and 9% MOG antibody disease, and 4% with another disease or have yet to receive a diagnosis.
35% were diagnosed less than one week after symptom onset, but for 32% of respondents it took longer than six weeks to be diagnosed. 83% of participants received treatment after their first acute attack. However, of those who received a first acute treatment, only 33% of participants received a second treatment that was different than the first. 76% of participants received rehabilitative therapy.
79% of participants currently have weakness or paralysis, 78% of participants have numbness or loss of sensation, and 62% of participants have spasticity or uncontrolled muscle spasms. 54% experience neck or back pain, 76% of participants experience neuropathic pain, and 69% of participants have bladder and/or bowel symptoms.
Quality of Life (QoL) and Ongoing Care Management Among those with Neuromyelitis Optica Spectrum Disorder (NMOSD)
The primary objective for this study is to understand the experience and perceptions of those with NMOSD or their care partners on their quality of life in relation to their ongoing management of care, including accessing medical care, treatments, and potential barriers experienced during the course of their lives in relation to their disorder.
We explored the following questions:
- How do those with NMOSD or their care partners perceive QoL?
- What does the ongoing care journey look like for those with NMOSD?
- How might we design new programs and services that improve the QoL of those with NMOSD in relation to ongoing care?
To our knowledge, there are no published qualitative studies that use patient reported outcomes (PROs) through a human-centered design process to identify the perceptions of QoL in people with rare neuroimmune disorders in relation to their ongoing care. The core tenets of human-centered design include the following sequential steps: empathize with all stakeholders; define the problem; ideate in an open-minded manner; prototype solutions; and test. Our application of human-centered design is based on the framework of Grounded Theory, in which themes are identified during a series of interviews, practical activities (e.g., a diary study), an ideation session, and a prototype review session. An ideation session is a process where ideas are generated through a group session that includes visual activities with others diagnosed with NMOSD. Understanding the triggers and perceptions of QoL is important for our work as an organization to best meet the needs of our patient community and co-develop solutions to improve ongoing care management.
We had a total of 58 people fill out the screener, and 12 were interviewed by study staff. We then held a total of 4 ideation sessions, three with individuals with NMOSD (n=11) and one with medical professionals who see patients with NMOSD (n=3). Based on the discussions in the ideation session, the study team created a “patient journey.” We shared this patient journey in prototyping sessions with 11 individuals with NMOSD and will be publishing the results